
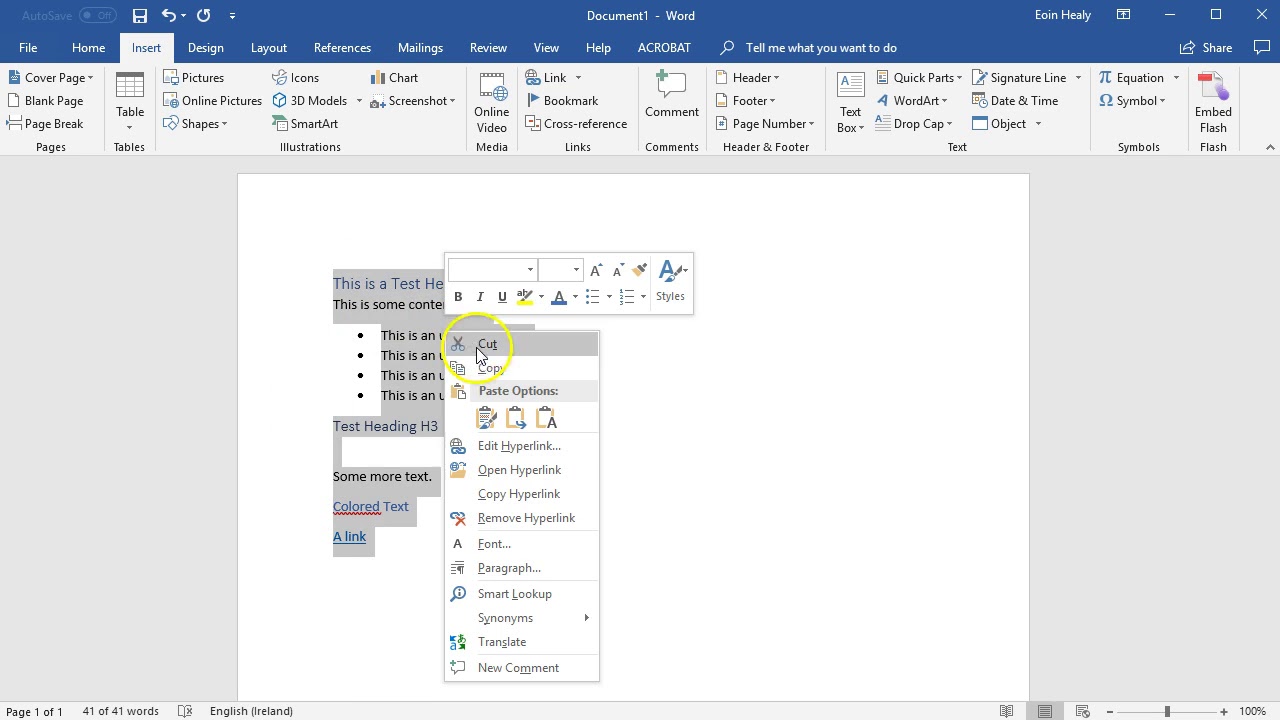
| |8 |Tc |405.5 | |9 |Z |0.8718268 | Nonlinear equations |1 |f(V) = (P+a/(V^2))*(V-b)-R*T = 0 | Explicit equations |1 |P = 56 | |2 |R = 0.08206 | |3 |T = 450 | |4 |Tc = 405.5 | |5 |Pc = 111.3 | |6 |Pr = P/Pc | |7 |a = 27*(R^2*Tc^2/Pc)/64 | |8 |b = R*Tc/(8*Pc) | |9 |Z = P*V/(R*T) | General Settings |Total number of equations |10 | |Number of implicit equations |1 | |Number of explicit equations |9 | |Elapsed time |0.0000 sec | |Solution method |SAFENEWT | |Max iterations |150 | |Tolerance F |0.0000001 | |Tolerance X |0.0000001 | |Tolerance min |0.0000001 | Figure 1: Polymath Plot for Van der Waals Problem Solve example 1 in the Ordinary Differential Equations Tutorial: In presenting the solution do the following and produce the printout found on the following page: 8. Van Der Waals Equation | |Nonlinear Equation |1 | Calculated values of NLE variables | |Variable |Value | |1 |a |4.196946 | |2 |b |0.0373712 | |3 |P |56. |POLYMATH Report |1.1(a) Molar Volume and Compress. You can either hand write the units to the axis or use the Microsoft drawing tools to add the units. You will need to edit the graph to label the axis to produce what is shown below. Then use select all and cut and paste the POLYMATH report into a word document as shown below as well as the graph. Note that for equation 1, this should be a sample calculation using your initial guess. Write this calculation on engineering paper as well your hand calculations with units for equations 1, 2, 3, and 5. Estimate the volume using the ideal gas law. In presenting the solution do the following:and produce the printout found on the following page: 1. This is taken from the Cutlip and Shacham text book titled, “Problem solving in Chemical Engineering with Numerical Methods.” The van der Waals equation of state is given by (1) Where (2) And (3) The variables are defined as: The reduced pressure is defined as (4) And th compressibility factor is given by (5) Calculate the molar volume and compressibility factor for gaseous ammonia at a pressure of 56 atm and a temperature of 450 K using the van der Waals equation of state. What did you type? Now review the Non linear Equations solver: Following Example 1 and Example 2 given in the Polymath program tutorial solve the following problem. Have polymath, using the calculator give you the cosine of 30 degrees. How would you have polymath give you the absolute value of a number? 7. What symbol on the tool bar represents the polymath scientific constants menu? Give the value of pi to an accuracy of 12 digits using polymath scientific constants.

What symbol on the tool bar represents the unit conversion calculator? Using the polymath unit conversion calculator convert 1 hp (international) to J/s. How many explicit equations can be solved using the POLYMATH ode solver? 3. How many simultaneous ordinary differential equations can be simultaneously solved using the educational version of POLYMATH? 2. Read the section titled Introduction to Polymath both getting started and Variables and expressions and answer the following questions: 1. Examine the Help menu by selecting the question mark or the word menu Help.

Select the Polymath program “Polymath EDU site”. Start the tutorial by opening the POLYMATH program which is usually found from the Chemical Apps section of the Start, Programs menu. POLYMATH TutorialĜhemical Reaction Engineering This tutorial will introduce you to the basics of Polymath.


 0 kommentar(er)
0 kommentar(er)
